PURPOSE: The World Trade Center (WTC) attack of September 11, 2001 created an unprecedented environmental exposure to known and suspected carcinogens. A higher incidence of multiple myeloma (MM) and precursor disease has been reported among first responders to the WTC disaster compared to the unexposed population (Landgren, 2018). To expand on prior screening studies, and to characterize the genomic impact of the exposure to known and potential carcinogens in the WTC debris, we were motivated to perform whole genome sequencing (WGS) of WTC first responders and recovery workers who were diagnosed with a plasma cell disorder after the attack.
PATIENTS AND METHODS: We performed WGS of 9 CD138-positive bone marrow mononuclear samples from patients who were diagnosed with plasma cell disorders after exposure to the WTC disaster: 4 monoclonal gammopathy of undetermined significance (MGUS), 2 smoldering multiple myeloma (SMM), 2 MMs, and 1 patient with plasma cell leukemia (PCL). Eight patients (88%) were first responders and one was a recovery worker. Peripheral blood mononuclear cells were used as normal match. Median coverage for tumor and normal samples was 50.9X (range 47-76) and 37X (range 35-41), respectively. The landscape of genomic drivers and complex structural events was compared to 752 MM patients enrolled in the CoMMpass trial with available whole exome and low-coverage long-insert WGS data (IA15; NCT01454297). To characterize the mutational signature landscape we combined the WTC cohort with 110 whole genomes from 56 patients with multiple myeloma and myeloma precursor disease (Rustad et al. 2020; Landau et al. 2020) and we ran our three-step workflow: de novo extraction (i.e. sigprofiler), assignment, and fitting (i.e. mmsig). To exclude contribution of any environmental agents in the WTC debris with known mutational signatures (Kucab et al., 2019), we ran our fitting algorithm mmsig in each post-WTC case, including and forcing the extraction of these mutational signatures.
RESULTS: No significant differences were observed in comparing the post-WTC driver and mutational signatures landscape with 110 previously published WGS from 56 patients with MM and the CoMMpass WGS cohort (n=752). Likewise, we did not observe any new or distinct mutational signatures among WTC-exposed patients. Following forced extraction of 5 mutational signatures associated with environmental agents detected in the WTC debris (e.g. PAHs), we did not find significant contributions from any of these described environmental mutational signatures.
To reconstruct the temporal activity of each mutational process we divided all single nucleotide variants into subclonal and clonal. Clonal mutations were further subdivided into duplicated (acquired before a chromosomal gain) and unduplicated (Rustad et al. 2020). WTC-exposed patients had differing patterns in mutational signature timelines of AID and APOBEC activity. Overall, the mutational signature activity over time in post-WTC plasma cell dyscrasia reflects what has been previously observed in multiple myeloma without WTC-exposure (Rustad et al., 2020).
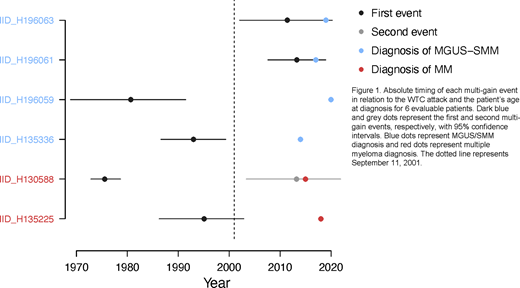
Finally, leveraging constant activity of the clock-like single base substitution mutational signatures 1 and 5 over time and our molecular time workflow (Rustad et al., 2020), we estimated the age at which each evaluable patient acquired a tumor-initiating chromosomal gain and found that they were windowed to both pre- and post-WTC exposure across neoplasms (Figure 1). In some cases, clonal multi-chromosomal gain events were acquired decades before both the diagnosis and the WTC exposure. Specifically, of 6 patients with large clonal chromosomal gains, 1 MM case, 1 SMM, and 1 MGUS showed evidence of a pre-existing clone prior to WTC exposure, two MGUS showed evidence of multi-gain events following the exposure, and one MM case had a 1q gain in the same time window as the attack.
CONCLUSIONS: Post-WTC plasma cell neoplasms had similar genomic landscapes to non-exposed cases. Although limitations in sample size preclude any definitive conclusions, our findings suggest that the observed increased incidence of plasma cell neoplasms in this population is due to complex and heterogeneous effects of the WTC exposure that may have initiated or contributed to progression of malignancy. The existence of pre-malignant clonal entities at time of WTC exposure may therefore be relevant for future WTC-related study.
Hultcrantz:Intellisphere LLC: Consultancy; Amgen: Research Funding; Daiichi Sankyo: Research Funding; GSK: Research Funding. Shah:Physicians Education Resource: Honoraria; Celgene/BMS: Research Funding. Iacobuzio-Donahue:BMS: Research Funding. Papaemmanuil:Isabl: Current equity holder in private company, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria; Illumina: Consultancy, Honoraria; Kyowa Hakko Kirin: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Prime Oncology: Consultancy, Honoraria; MSKCC: Patents & Royalties. Verma:BMS: Consultancy, Research Funding; acceleron: Consultancy, Honoraria; stelexis: Current equity holder in private company; Janssen: Research Funding; Medpacto: Research Funding. Dogan:National Cancer Institute: Research Funding; EUSA Pharma: Consultancy; Takeda: Consultancy; Seattle Genetics: Consultancy; Corvus Pharmaceuticals: Consultancy; Physicians Education Resource: Consultancy; Roche: Consultancy, Research Funding; AbbVie: Consultancy. Landgren:Celgene: Consultancy, Honoraria, Research Funding; Seattle Genetics: Research Funding; Cellectis: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Merck: Other; Karyopharma: Research Funding; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Pfizer: Consultancy, Honoraria; Merck: Other; Karyopharma: Research Funding; Binding Site: Consultancy, Honoraria; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Seattle Genetics: Research Funding; Takeda: Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Janssen: Consultancy, Honoraria, Other: Independent Data Monitoring Committees for clinical trials, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria; Juno: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Adaptive: Consultancy, Honoraria; Cellectis: Consultancy, Honoraria; Glenmark: Consultancy, Honoraria, Research Funding; Binding Site: Consultancy, Honoraria; BMS: Consultancy, Honoraria; Glenmark: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal